Annotation Enrichment, Clustering and dimensionality reduction
A python notebook with steps to do Annotation Enrichment Analysis (hypergeometric test) and different clustering and dimensionality reduction methods on a proteomics dataset
View this project in github
#Gene Annotation Enrichment #################################################################################################
def enrich(input_gene_list, background_gene_list):
category_f = "/home/vitalv/cyano/category.txt"
category_df = pd.read_csv(category_f, delimiter="\t", header=None, \
names=["geneID", "definition", "first_category_ID", "first_category_name", "second_category_ID", "second_category_name"])
data = category_df[category_df["geneID"].isin(input_gene_list)]
background = category_df[category_df["geneID"].isin(background_gene_list)]
print "first_category_annotation" + "\t" + "Genes in input list w/ annotation" + "\t" + "Genes in input list" + \
"\t" + "Genes in background list w/ annotation" + "\t" + "Genes in background list" + "\t" + "p-value"
for category in data.first_category_name.unique():
k = len(data) #input list (list of differentially abundant proteins -ANOVA<0.05-)
x = len(data[data.first_category_name == category]) #num of genes in the input list with the annotation
N = len(set(background_gene_list)) #background number of genes.
m = len(background[background.first_category_name == category])
p = stats.hypergeom.sf(x, N, k, m)
print category + "\t" + str(x) + "\t" + str(k) + "\t" + str(m) + "\t" + str(N) + "\t" + str(p)
enrich(list(data.geneID), set(all_prots))
| first_category_annotation | Genes in input list w/ annotation | Genes in input list | Genes in background list w/ annotation | Genes in background list | p-value |
| Amino acid biosynthesis | 60 | 973 | 91 | 2717 | 7.35140656231e-10 |
| Biosynthesis of cofactors, prosthetic groups, and carriers | 47 | 973 | 113 | 2717 | 0.0804178252749 |
| Cell envelope | 37 | 973 | 59 | 2717 | 5.95410352239e-06 |
| Cellular processes | 40 | 973 | 66 | 2717 | 9.66099919551e-06 |
def correct_pvalues_for_multiple_testing(pvalues, correction_type = "Benjamini-Hochberg"):
pvalues = np.array(pvalues)
n = float(pvalues.shape[0])
new_pvalues = np.empty(pvalues.shape[0])
if correction_type == "Bonferroni":
new_pvalues = n * pvalues
elif correction_type == "Bonferroni-Holm":
values = [ (pvalue, i) for i, pvalue in enumerate(pvalues) ]
values.sort()
for rank, vals in enumerate(values):
pvalue, i = vals
new_pvalues[i] = (n-rank) * pvalue
elif correction_type == "Benjamini-Hochberg":
values = [ (pvalue, i) for i, pvalue in enumerate(pvalues) ]
values.sort()
values.reverse()
new_values = []
for i, vals in enumerate(values):
rank = n - i
pvalue, index = vals
new_values.append((n/rank) * pvalue)
for i in xrange(0, int(n)-1):
if new_values[i] < new_values[i+1]:
new_values[i+1] = new_values[i]
for i, vals in enumerate(values):
pvalue, index = vals
new_pvalues[index] = new_values[i]
return new_pvalues
def enrichGO(input_gene_list, background_gene_list, ontology):
'''
#ontology is one of : Biological Process, Molecular Function or Cellular Component
'''
go_terms_f = "/home/vitalv/cyano/label-free-demixq-diffacto-pipeline/goterm.txt"
go_df = pd.read_csv(go_terms_f, delimiter="\t", header=None, names=["species", "geneID", "annotation_type", "go_term"])
data_go = go_df[go_df["geneID"].isin(input_gene_list)]
data = data_go[data_go.go_term.str.contains(ontology, case=False)]
background = go_df[go_df["geneID"].isin(set(all_prots))]
background = background[background.go_term.str.contains(ontology, case=False)]
#print "GO_annotation" + "\t" + "Genes in input list w/ annotation" + "\t" + "Genes in input list" + "\t" \
#+ "Genes in background list w/ annotation" + "\t" + "Genes in background list" + "\t" + "p-value"
enrichGO_pvalues = []
with open("enrichGO.txt", "wb") as f:
f.write("GO_annotation" + "\t" + "Genes in input list w/ annotation" + "\t" + "Genes in input list" + "\t" + \
"Genes in background list w/ annotation" + "\t" + "Genes in background list" + "\t" + "p-value" + "\n")
for annotation in data.go_term.unique():
k = len(data.geneID.unique())
x = len(data[data.go_term == annotation])
N = len(set(all_prots))
m = len(background[background.go_term == annotation])
p = stats.hypergeom.sf(x, N, k, m)
#print annotation + "\t" + str(x) + "\t" + str(k) + "\t" + str(m) + "\t" + str(N) + "\t" + str(p)
enrichGO_pvalues.append(p)
f.write(annotation + "\t" + str(x) + "\t" + str(k) + "\t" + str(m) + "\t" + str(N) + "\t" + str(p) + "\n")
return enrichGO_pvalues
import statsmodels.sandbox.stats.multicomp as mc
from IPython.display import display, HTML
enrichGO_pvalues = enrichGO(list(data.geneID), set(all_prots), "biological process")
enrichGO_df = pd.read_csv("enrichGO.txt", delimiter="\t")
'''
http://statsmodels.sourceforge.net/devel/generated/statsmodels.sandbox.stats.multicomp.multipletests.html
statsmodels.sandbox.stats.multicomp.multipletests(pvals, alpha=0.05, method='hs', is_sorted=False, returnsorted=False)
method is one of:
`bonferroni` : one-step correction
`sidak` : one-step correction
`holm-sidak` : step down method using Sidak adjustments
`holm` : step-down method using Bonferroni adjustments
`simes-hochberg` : step-up method (independent)
`hommel` : closed method based on Simes tests (non-negative)
`fdr_bh` : Benjamini/Hochberg (non-negative)
`fdr_by` : Benjamini/Yekutieli (negative)
`fdr_tsbh` : two stage fdr correction (non-negative)
`fdr_tsbky` : two stage fdr correction (non-negative)
'''
reject, adj_pvalues, corrected_a_sidak, corrected_a_bonf = mc.multipletests(enrichGO_df["p-value"], method='fdr_bh')
enrichGO_df["adj_pval"] = adj_pvalues
HTML(enrichGO_df.to_html())
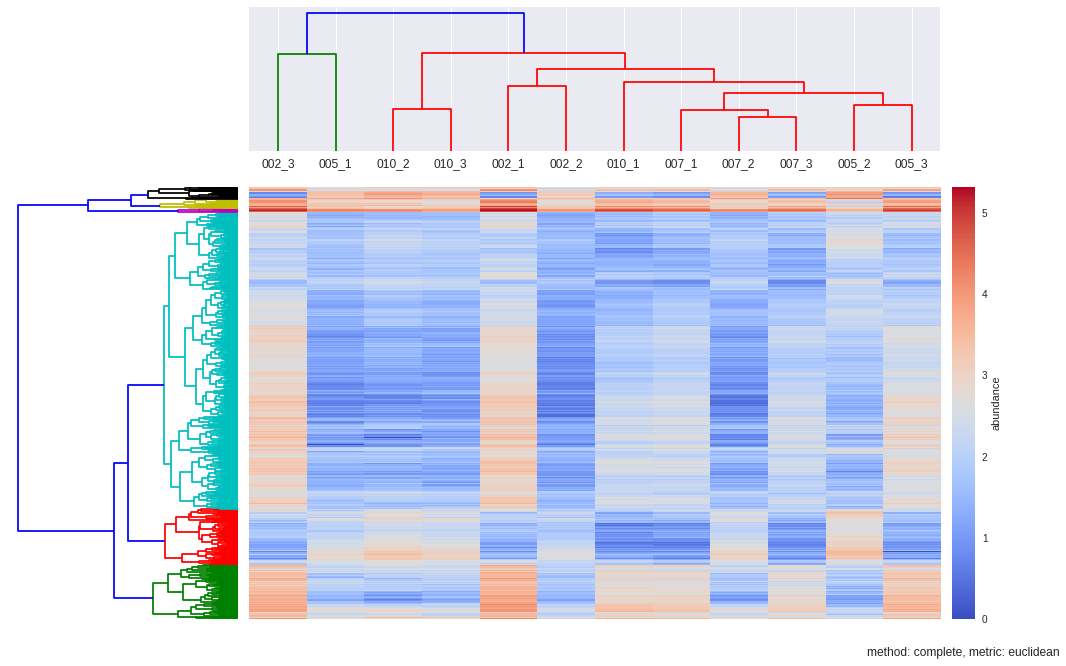
Hierarchical Clustering
for the point:
(xa, ya, za)
(xb, yb, zb)
the euclidean distance is:
euclidean_dist = sqrt((xa-xb)^2 + (ya-yb)^2 + (za-zb)^2)
pdist is equivalent to : [np.sqrt(np.sum((matrix[0]-matrix[1])^2)), np.sqrt(np.sum((matrix[0]-matrix[2])^2)), … ]
#HIERARCHICAL CLUSTER ANALYSYS #############################################################################################
import scipy.cluster.hierarchy as sch
import scipy.spatial.distance as dist
#diffacto_out = "/home/vitalv/cyano/label-free-demixq-diffacto-pipeline/diffacto_out.tsv"
#diffacto = pd.read_csv(diffacto_out, delimiter="\t")
#data = diffacto[diffacto["S/N"] > -20]
#samples = ["002_1", "002_2", "002_3", "005_1", "005_2", "005_3", "007_1", "007_2", "007_3", "010_1", "010_2", "010_3"]
#convert back to linear space:
#data[samples] = 2 ** data[samples]
matrix = 2**data[samples].values
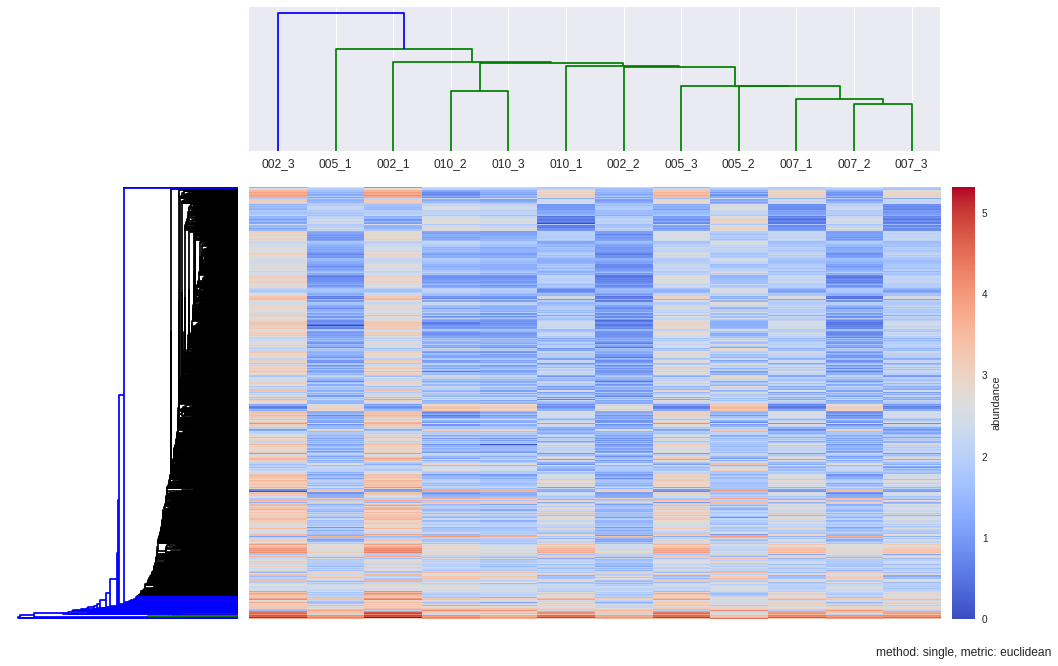
def hcluster(matrix, method='complete', metric='euclidean', dendro_color_threshold=0.45):
fig = plt.figure(figsize=(16,10))
#First dendrogram. Rows. Genes.
D = dist.pdist(matrix, metric=metric) #Pairwise distances between observations in n-dimensional space.
D = dist.squareform(D)
ax1 = fig.add_axes([0.09,0.1,0.2,0.6])
Y = sch.linkage(D, method=method, metric=metric)
Z1 = sch.dendrogram(Y, orientation='left', color_threshold=dendro_color_threshold*max(Y[:,2]))
#colors all descendent links below a cluster node k the same color if k is the first node below the cut threshold
ax1.set_xticks([])
ax1.set_yticks([])
ax1.axis('off')
#Second dendrogram. Columns. Samples
D_samples = dist.pdist(matrix.T, metric=metric)
D_samples = dist.squareform(D_samples)
ax2 = fig.add_axes([0.3,0.75,0.6,0.2])
Y = sch.linkage(D_samples, method=method, metric=metric)
#for label in ax2.get_xticklabels(): label.set_rotation(45)
Z2 = sch.dendrogram(Y, labels=samples, color_threshold=max(Y[:,2]))
#ax2.set_xticks([])
ax2.set_yticks([])
#remove outer border(frame)
ax2.spines['top'].set_visible(False)
ax2.spines['right'].set_visible(False)
ax2.spines['bottom'].set_visible(False)
ax2.spines['left'].set_visible(False)
# Scale the max and min colors so that 0 is white/black
cmap=plt.cm.coolwarm #plt.cm.YlGnBu
vmin = matrix.min()
vmax = matrix.max()
vmax = max([vmax,abs(vmin)])
vmin = vmax*-1
norm = matplotlib.colors.Normalize(vmin/2, vmax/2)
# Plot heatmap
axmatrix = fig.add_axes([0.3,0.1,0.6,0.6])
idx1 = Z1['leaves']
idx2 = Z2['leaves']
D = D[idx1,:]
D = D[:,idx2]
heatmap = axmatrix.matshow(D, aspect='auto', origin='lower', cmap=cmap)#, norm=norm) #use norm if values are log
axmatrix.set_xticks([])
axmatrix.set_yticks([])
heatmap
# Plot colorbar.
axcolor = fig.add_axes([0.91,0.1,0.02,0.6])
colorbar = plt.colorbar(heatmap, cax=axcolor)
colorbar.set_label("abundance")
plt.title("method: %s, metric: %s"%(method,metric), x=0.5, y=-0.1)
return Z1, Z2, heatmap
dendroRows_com_euc, dendroCols, heatmap = hcluster(matrix, method='complete', metric='euclidean', dendro_color_threshold=0.45)
#OTHER DENDROGRAM METHODS AND METRICS
dendroRows_sin_euc, dendroCols, heatmap = hcluster(matrix, method='single', metric='euclidean', dendro_color_threshold=0.45)
dendroRows_com_cos, dendroCols, heatmap = hcluster(matrix, method='complete', metric='cosine', dendro_color_threshold=0.45)

K Means
separate samples in n groups of equal variance
first step chooses the initial centroids (k, the number of clusters)
After initialization, K-means consists of looping between the two other steps:
The first step assigns each sample to its nearest centroid.
The second step creates new centroids by taking the mean value of all of the samples assigned to each previous centroid
The inertia or within-cluster sum-of-squares is minimized
#OTHER CLUSTERING METHODS BESIDES HCL: K-MEANS AND MEAN SHIFT ##############################################################
from sklearn.cluster import KMeans
from scipy.spatial.distance import pdist,cdist
#diffacto_out = "/home/vitalv/cyano/label-free-demixq-diffacto-pipeline/diffacto_out.tsv"
#diffacto = pd.read_csv(diffacto_out, delimiter="\t")
#data = diffacto[diffacto["S/N"] > -20]
#samples = ["002_1", "002_2", "002_3", "005_1", "005_2", "005_3", "007_1", "007_2", "007_3", "010_1", "010_2", "010_3"]
#data[samples] = 2 ** data[samples] #convert back to linear space, diffacto output is log2
matrix = 2**data[samples].values
#K-MEANS
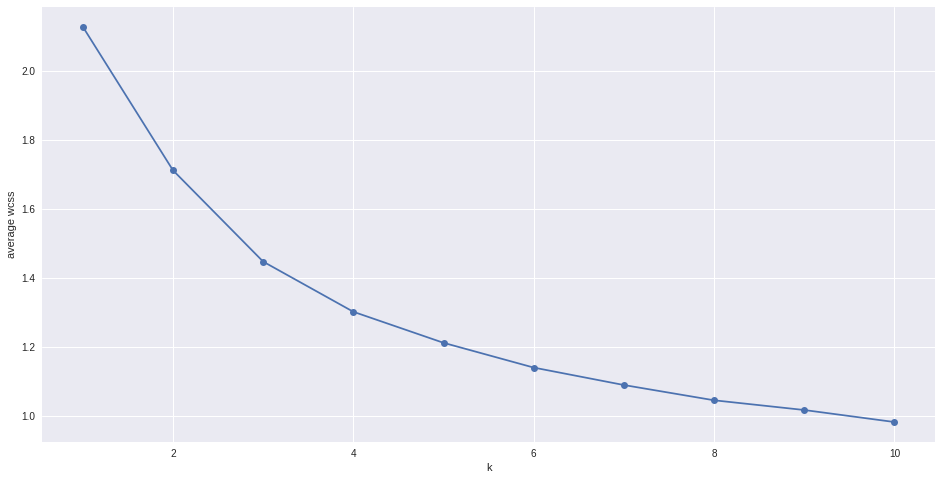
# Try to find optimal number of clusters for k-means. "Elbow" method
k_range = range(1,11)
k_means = [KMeans(n_clusters = k, init='k-means++').fit(matrix) for k in k_range]
centroids = [X.cluster_centers_ for X in k_means]
labels = [X.labels_ for X in k_means]
#calculate Euclidean distance from each point to cluster center
k_euclid = [cdist(matrix, c, 'euclidean') for c in centroids]
dist = [np.min(ke, axis=1) for ke in k_euclid]
#Total within cluster sum of squares
wcss = [sum(d**2) for d in dist]
#average wcss
avwcss = [(sum(d**2))/len(d) for d in dist]
#total sum of squares
tss = sum(pdist(matrix)**2)/matrix.shape[0]
#between cluster sum of squares:
bss = tss - wcss
#plot average wcss vs number of clusters "Elbow plot": look for a point where the rate of decrease in wcss sharply shifts
plt.plot(k_range, avwcss, '-o')
plt.ylabel("average wcss")
plt.xlabel("k")
<matplotlib.text.Text at 0x7fd32a0e7510>
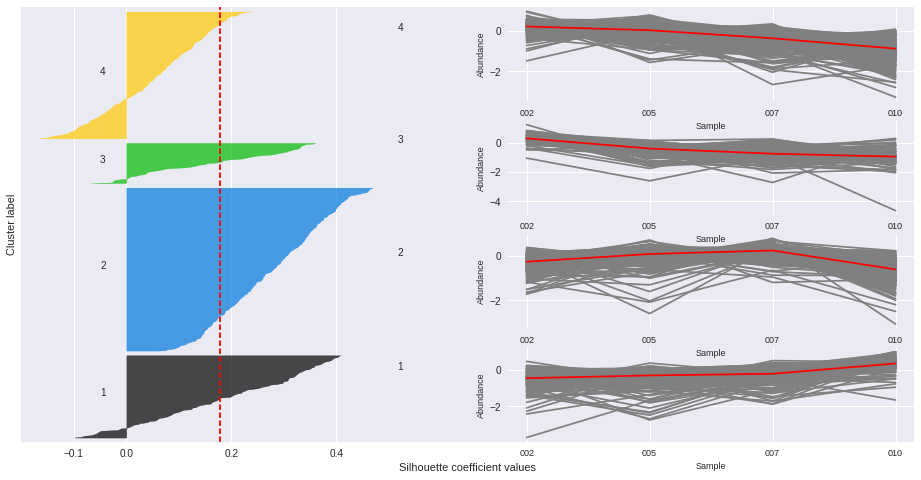
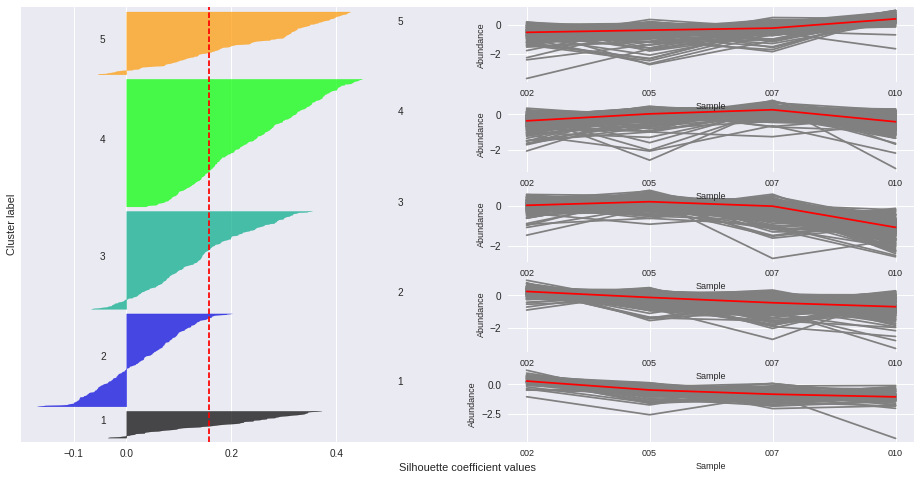
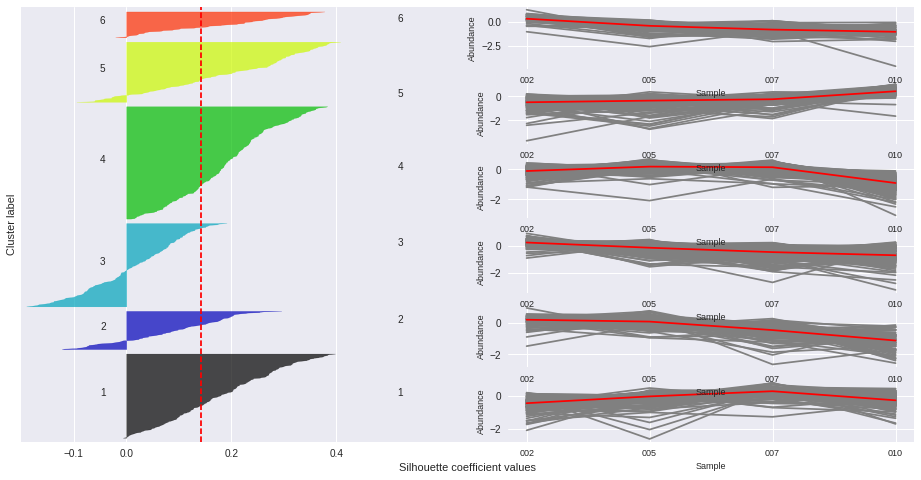
Silhouette plots
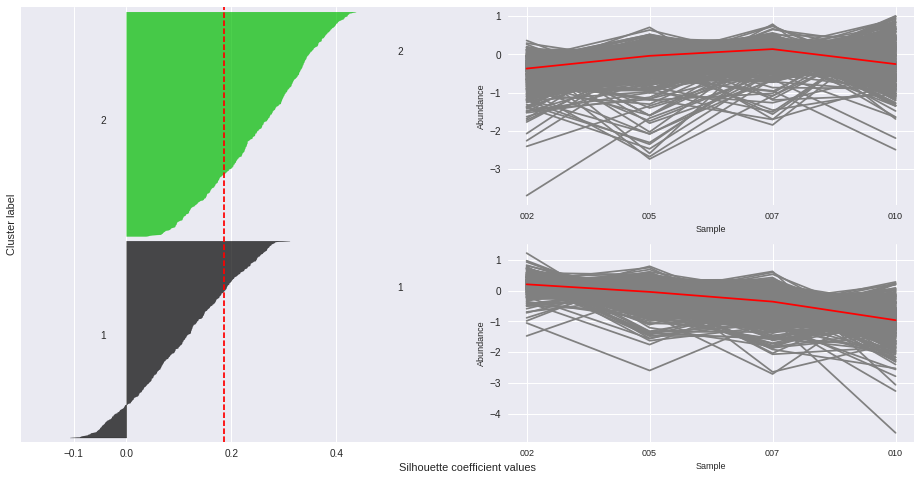
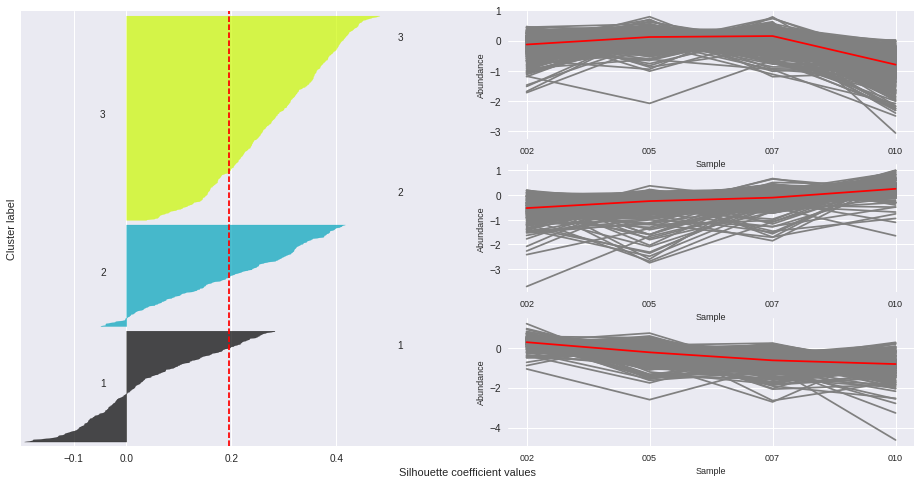
The silhouette plot displays a measure of how close each point in one cluster is to points in the neighboring clusters. Provides a way to assess parameters like number of clusters visually. This measure has a range of [-1, 1].
Silhouette coefficients (as these values are referred to as) near +1 indicate that the sample is far away from the neighboring clusters.
A value of 0 indicates that the sample is on or very close to the decision boundary between two neighboring clusters
and negative values indicate that those samples might have been assigned to the wrong cluster.
The Silhouette Coefficient is calculated using the mean intra-cluster distance (a) and the mean nearest-cluster distance (b) for each sample. The Silhouette Coefficient for a sample is (b - a) / max(a, b)
def cluster_abundance(protein_idxs, ax=None):
protein_set = data[data["Protein"].isin(data.iloc[idxs, :].Protein)]
X = [1,2,3,4]
x_labels = set([c.split("_")[0] for c in samples])
nCols = len(X)
color_patches = []
if not ax: fig, ax = plt.subplots()
color=iter(plt.cm.rainbow(np.linspace(0,1,len(protein_set))))
for i, row in protein_set.iterrows():
Ys = row[samples].values.reshape(4,3).T #for scatter
nRows = Ys.shape[0]
Xs=X*nRows
ax.plot(X, row[means].values, "-", color='gray')
medians = protein_set[means].median(axis=0)
ax.plot(X, medians, "-", color='red')
ax.set_ylabel("Abundance", fontsize=9)
ax.set_xlabel("Sample", fontsize=9)
ax.set_title("", fontsize=10)
ax.set_xticks(X)
ax.set_xticklabels(sorted(list(x_labels)), fontsize=9)
#Silhouette coefficients and cluster abundance plots ------------------------------------------------------------------------
import matplotlib.cm as cm
from sklearn.metrics import silhouette_score, silhouette_samples
range_n_clusters = range(2,7)
for n_clusters in range_n_clusters:
fig, ax1 = plt.subplots(figsize=(16,8))
#fig, (ax1, ax2) = plt.subplots(1, 2) # Create a subplot with 1 row and 2 columns
ax1.set_xlim([-0.2, 1.5])
ax1.set_ylim([0, len(matrix) + (n_clusters + 1) * 10]) #inserts blank space between silhouette plots of individual cluster
# Initialize the clusterer with n_clusters value and a random generator seed of 10 for reproducibility.
kmeans = KMeans(n_clusters=n_clusters, init='k-means++')#, random_state=10)
cluster_labels = kmeans.fit_predict(matrix)
silhouette_avg = silhouette_score(matrix, cluster_labels) # The silhouette_score gives the average value for all the samples.
print "n_clusters:", n_clusters, "Average silhouette_score is :", silhouette_avg
sample_silhouette_values = silhouette_samples(matrix, cluster_labels) # Compute the silhouette scores for each sample
cluster_idxs = {}
y_lower = 10
for i in range(n_clusters):
c = cluster_labels == cluster
cluster_idxs[cluster] = np.where(c == True)
ith_cluster_silhouette_values = sample_silhouette_values[cluster_labels == i]# Aggregate silhouette scores
ith_cluster_silhouette_values.sort() # and sort them
size_cluster_i = ith_cluster_silhouette_values.shape[0]
y_upper = y_lower + size_cluster_i
color = cm.spectral(float(i) / n_clusters)
ax1.fill_betweenx(np.arange(y_lower, y_upper), 0, ith_cluster_silhouette_values, facecolor=color, edgecolor=color, alpha=0.7)
ax1.text(-0.05, y_lower + 0.5 * size_cluster_i, str(i+1)) # Label the silhouette plots with their cluster numbers at the middle
y_lower = y_upper + 10 # # Compute the new y_lower for next plot 10 for the 0 samples
position=range(n_clusters)[-(i+1)]*2+2
ax_n = fig.add_subplot(n_clusters,2,position) #add_subplot(rows,columns,positionInGrid)
ax_n.text(-0.05,0,str(i+1))
idxs = list(cluster_idxs[i][0])
cluster_abundance(idxs, ax=ax_n)
ax1.set_xlabel("Silhouette coefficient values")
ax1.set_ylabel("Cluster label")
ax1.axvline(x=silhouette_avg, color="red", linestyle="--")# Vertical line for average silhouette score of all the values
ax1.set_yticks([]) # Clear the yaxis labels / ticks
ax1.set_xticks([-0.1, 0, 0.2, 0.4])
n_clusters: 2 Average silhouette_score is : 0.185305484063
n_clusters: 3 Average silhouette_score is : 0.195025791161
n_clusters: 4 Average silhouette_score is : 0.178523494958
n_clusters: 5 Average silhouette_score is : 0.158383531414
n_clusters: 6 Average silhouette_score is : 0.14186245441
Mean Shift
Mean shift has the advantage that it does not require a pre-defined number of clusters
Updates centroid candidates in each iteration so they become the mean of the points within a region of size determined by the paramater bandwidth
The mean shift vector is computed for each centroid and points towards a region of the maximum increase in the density of points
from sklearn.cluster import MeanShift, estimate_bandwidth
bandwidth = estimate_bandwidth(matrix, quantile=0.3, n_samples=980) #default: quantile=0.3, n_samples= (all samples are used)
#quantile=0.5 means that the median of all pairwise distances is used
#but it takes a default value if bandwidth is not set
ms = MeanShift(bandwidth=bandwidth)
ms.fit(matrix)
labels = ms.labels_
cluster_centers = ms.cluster_centers_
labels_unique = np.unique(labels)
n_clusters_ = len(labels_unique)
cluster_idxs = {}
for cluster in range(n_clusters_):
c = labels == cluster
cluster_idxs[cluster] = np.where(c == True)
#cluster_idxs
Mean shift results in very un-evenly sized clusters with one of them containing the majority of proteins
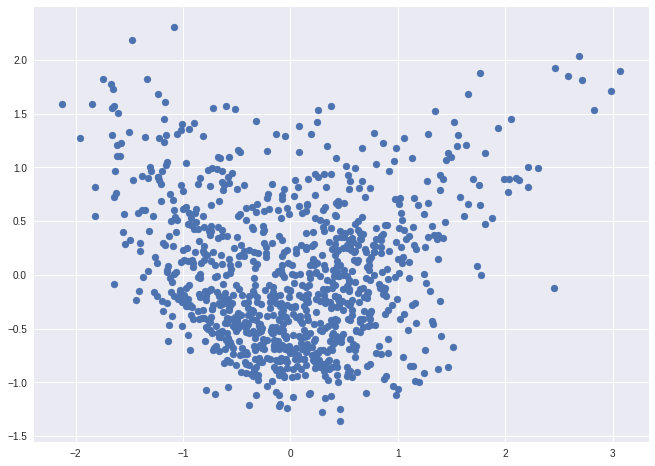
Dimensionality Reduction
PCA
from sklearn.decomposition import PCA
pca = PCA().fit_transform(matrix)
ax = plt.subplot(aspect='equal')
ax.scatter(pca[:,0], pca[:,1])
<matplotlib.collections.PathCollection at 0x7fd329fd23d0>
tSNE
tSNE is a dimensionality reduction technique useful for visualizing high-dimensional data
http://scikit-learn.org/stable/modules/generated/sklearn.manifold.TSNE.html#sklearn.manifold.TSNE
https://www.oreilly.com/learning/an-illustrated-introduction-to-the-t-sne-algorithm
The learning rate can be a critical parameter. It should be between 100 and 1000. If the cost function increases during initial optimization, the early exaggeration factor or the learning rate might be too high. If the cost function gets stuck in a bad local minimum increasing the learning rate helps sometimes
random_state is Pseudo Random Number generator seed control.
If None, use the numpy.random singleton. Note that different initializations might result in different local minima of the cost function.
from sklearn.manifold import TSNE
#defaults:
#tsne = TSNE(n_components=2, perplexity=30, learning_rate=1000, n_iter=1000) #learning_rate is also called epsilon
RS = 20150101#random_state is Pseudo Random Number generator seed control.
#If None, use the numpy.random singleton. Note that different initializations might result in different local minima of the cost function.
#tsne = TSNE(random_state=RS).fit_transform(matrix)
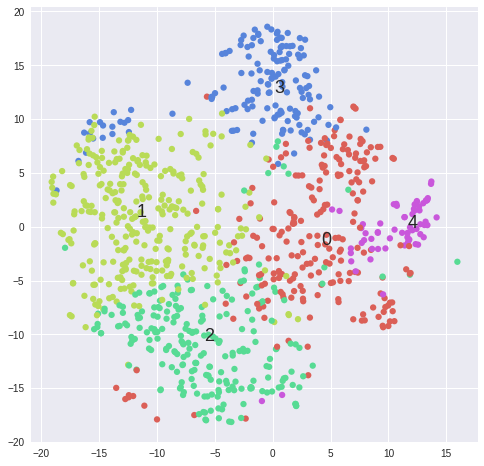
#Plot first_category_name---------------------------------------------------------------------------------
matrix = data.sort("first_category_name")[samples].values
labels = np.array(data.sort("first_category_name").first_category_name)
tsne_rs = TSNE(n_components=2, perplexity=30, learning_rate=1000, n_iter=1000, random_state=RS, verbose=1).fit_transform(matrix)
tsne_cos = TSNE(n_components=2, perplexity=30, learning_rate=1000, n_iter=1000, metric='cosine', verbose=1).fit_transform(matrix)
label_color_ = {}
for i in range(len(np.unique(labels))):
label = np.unique(labels)[i]
label_color_[label] = i
colors = np.array([label_color_[label] for label in labels])
def scatter(x, colors, nclasses):
palette = np.array(sb.color_palette("hls", nclasses )) # color palette with seaborn.
f = plt.figure(figsize=(8, 8))
ax = plt.subplot(aspect='equal')
sc = ax.scatter(x[:,0], x[:,1], linewidth=0, s=40, color=palette[colors.astype(np.int)])
plt.xlim(-25, 25)
plt.ylim(-25, 25)
#ax.axis('off')
ax.axis('tight')
txts = []
for i in range(nclasses):
# Position of each label.
xtext, ytext = np.median(x[colors == i, :], axis=0)
txt = ax.text(xtext, ytext, str(i), fontsize=18)
#txt.set_path_effects([
# PathEffects.Stroke(linewidth=5, foreground="w"),
# PathEffects.Normal()])
txts.append(txt)
return f, ax, sc, txts
scatter(tsne_cos, colors, len(set(colors)))
[t-SNE] Computing pairwise distances...
[t-SNE] Computing 91 nearest neighbors...
[t-SNE] Computed conditional probabilities for sample 980 / 980
[t-SNE] Mean sigma: 0.617742
[t-SNE] KL divergence after 100 iterations with early exaggeration: 1.077683
[t-SNE] Error after 200 iterations: 1.077683
[t-SNE] Computing pairwise distances...
[t-SNE] Computing 91 nearest neighbors...
[t-SNE] Computed conditional probabilities for sample 980 / 980
[t-SNE] Mean sigma: 0.197319
[t-SNE] KL divergence after 100 iterations with early exaggeration: 1.119585
[t-SNE] Error after 175 iterations: 1.119585
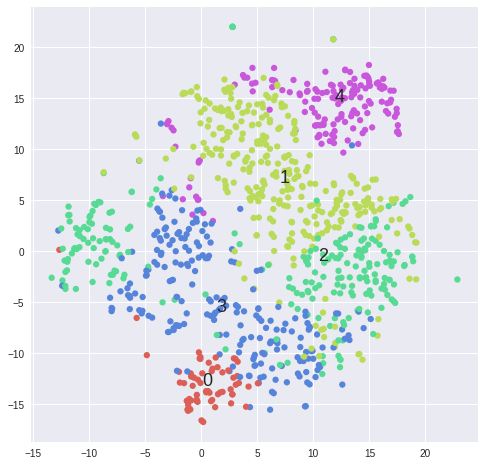
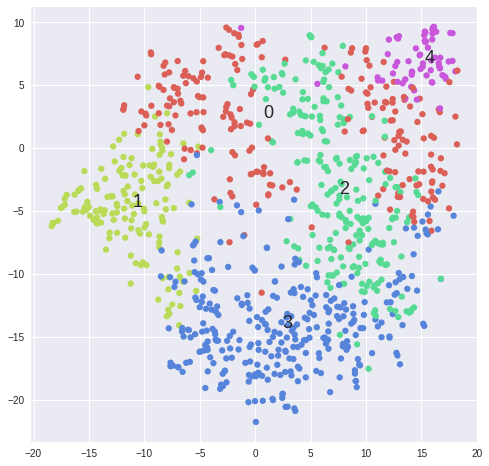
#Plot clusters from k-means ------------------------------------------------------------------------------------------------
matrix = 2**data[samples].values
for k in range(2,5):
kmeans = KMeans(n_clusters=5, init='k-means++')#, random_state=10)
cluster_labels = kmeans.fit_predict(matrix)
tsne = TSNE(n_components=2, perplexity=30, learning_rate=1000, n_iter=1000, metric='cosine', verbose=0).fit_transform(matrix)
scatter(tsne, cluster_labels, len(set(cluster_labels)))